The increasing use of antiglaucomatous drugs active on the uveo-scleral pathways (mainly prostaglandins and prostanoids) has in recent years renewed interest in the pathophysiological study of these outflow pathways.
As is well known, most of the aqueous humour produced is drained via a pressure-dependent pathway, at the level of the trabecularis; it is estimated that about 70-80 % of intra-ocular fluid is eliminated, under physiological conditions, via this pathway. The remaining 20-30% instead follows an alternative, pressure-independent pathway, reaching the suprachoroid after permeating the extracellular matrix of the ciliary body. Once in the suprachoroidal space, a (major) portion of aqueous humour re-enters the circulation by reabsorption by the choriocapillary via colloid-osmotic pressure gradients. A non-negligible part of fluid manages, however, to permeate the sclera and escape from the eyeball through the perineural and perivascular spaces of the episclera and thus the extra-orbital fatty tissues.2. The suprachoroidal space therefore assumes an intermediate position in this pathway as, ultimately, the aqueous humour flows through the ciliary muscle and then permeates the sclera from the suprachoroid. In most structures of the body, extra-vascular intra-tissue fluids are reabsorbed via the lymphatic vascular system; the absence of such a capillary network explains why alternative drainage pathways are activated in the sclera instead.
Recent studies
3 conducted on mouse eyes have experimentally verified these findings by studying the aqueous pathways by means of the intra-cameral introduction of fluorescent tracers (70-KDa dextran): from 60' after infusion, signs of trans-scleral filtration can clearly be appreciated. It is clear from the above that the sclera ultimately possesses its own hydraulic conductivity (understood as the ability to let fluids pass through it), which is fundamental in guaranteeing the completion of the trans-scleral outflow and clinically relevant for the implications it may have on the regulation of IOP (intra-ocular pressure) and the processes of absorption and elimination of drugs. With reference to this last aspect, it is easy to understand how the existence of a fluid flow directed from the inside to the outside that crosses the sclera, can hinder the absorption of drugs instilled locally, or through iontophoresis, osmotic pumps and peri-ocular injections
4,5. On the other hand, this phenomenon would seem to favour the elimination of drugs within the eyeball. The scleral hydraulic conductivity is influenced by various parameters, the most important of which are age and tissue thickness: generally an inverse proportionality relationship is observed with the former and a direct proportionality relationship with the latter, whereby the younger and thicker the sclera, the less resistance it offers to the passage of water. This is because a thick sclera will have larger spaces between the collagen laminae, greater hydration and therefore a lower hydraulic resistivity index. These aspects are in agreement with existing observations in the literature showing that the uveo-scleral outflow is greater in young people, that hydration levels are higher in young donors, and that the sclera is more compliant in surgeries performed on children's eyes. However, unexpectedly, hydraulic conductivity does not vary topographically, as would be expected as a result of the different scleral thickness between the anterior, equatorial and posterior portion
6On this theoretical basis, it is easy to see how an accurate knowledge and analysis of the hydro-conductive capacity of the sclera can provide new approaches to managing intra-ocular pressure in patients with ocular hypertension or glaucoma.
Importance of trans-scleral outflow in ocular hypertension and glaucoma
What happens to trans-scleral outflow in patients with ocular hypertension (OH) or primary open-angle glaucoma (POAG)? In recent years, it has become apparent that it is possible to study trans-scleral aqueous filtration in vivo, by analysing the microscopic characteristics of the filtering drafts in patients undergoing perforating surgery7,8by performing confocal microscopy of the conjunctiva. In working drafts (clinically of diffuse or cystic appearance), the presence of percolation and thus flow of aqueous permeating first the sclera and subsequently the conjunctival layers, is evidenced by the finding of intra-epithelial microcystic structures (Fig. 1).
[caption id="attachment_1892" align="alignleft" width="150"]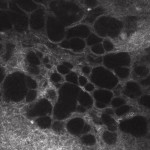 Ph 1: Functioning conjunctival draft: evidence of numerous epithelial microcysts, partly confluent[/caption].
Ph 1: Functioning conjunctival draft: evidence of numerous epithelial microcysts, partly confluent[/caption].
In contrast, in non-functional (clinically encapsulated or flat) drafts, few or no microcysts are observed, testifying to the mechanical obstacle to percolation provided by the subconjunctival fibrosis. Such cystic structures ultimately represent an in vivo sign of trans-scleral fluid passage. Recently, however, when studying the conjunctiva of ocular hypertensive patients and glaucomatous patients who are candidates for surgery, the presence of the same microcystic structures described in working drafts, albeit with a slightly different morphology, was found. The aim of the study9 was to assess and describe in vivo, with the aid of confocal microscopy, the existence and characteristics of trans-scleral outflow in untreated ocular hypertensive patients and in glaucomatous patients undergoing medical therapy.
Features and results of the study
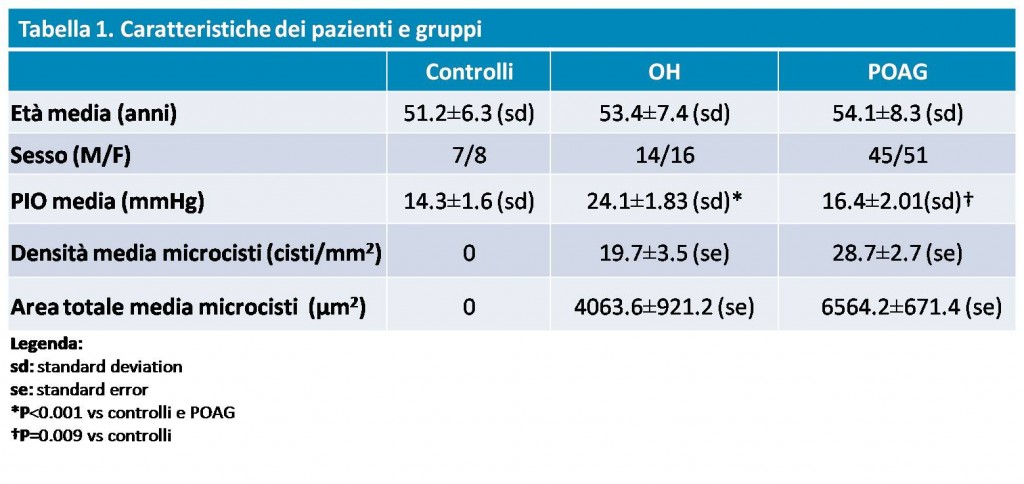
Thirty OH eyes not on drug therapy (IOP: 24.1± 1.83 mmHg) and 96 POAG eyes in pharmacological compensation (IOP: 16.4±2.01mmHg) were enrolled in the study (28 were taking ?-blockers as monotherapy, Group 1; 46 prostaglandins/prostamides as monotherapy, Group 2; 22 were on non-fixed combination therapy prostaglandins/prostamides + ?-blockers, Group 3); a group of 15 consecutive healthy eyes (IOP: 14.3±1.6 mmHg) was used as control. All patients underwent examination of the superior bulbar and temporal conjunctiva by in vivo confocal microscopy (LSM; HRT II Cornea Module; Heidelberg Engineering GmbH, Heidelberg, Germany).
[caption id="attachment_1893" align="alignleft" width="150"]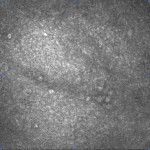 Ph 2: Control eye: no microcysts can be seen in the conjunctival epithelium[/caption].
Ph 2: Control eye: no microcysts can be seen in the conjunctival epithelium[/caption].
Two parameters were considered: the average density (cysts/mm2) and the total surface area of the microcysts (µm2), the latter calculated using ImageJ, an open source software available online (http://rsb.info.nih. gov/ij/).
The results obtained and the characteristics of the groups are shown in Table 1.
As can be seen from the data, microcystic structures are absent in normal eyes (Fig. 2), whereas they are noticeable in all hypertensive (Fig. 3) and glaucomatous (Fig. 4) eyes, with no significant differences between OH and POAG.
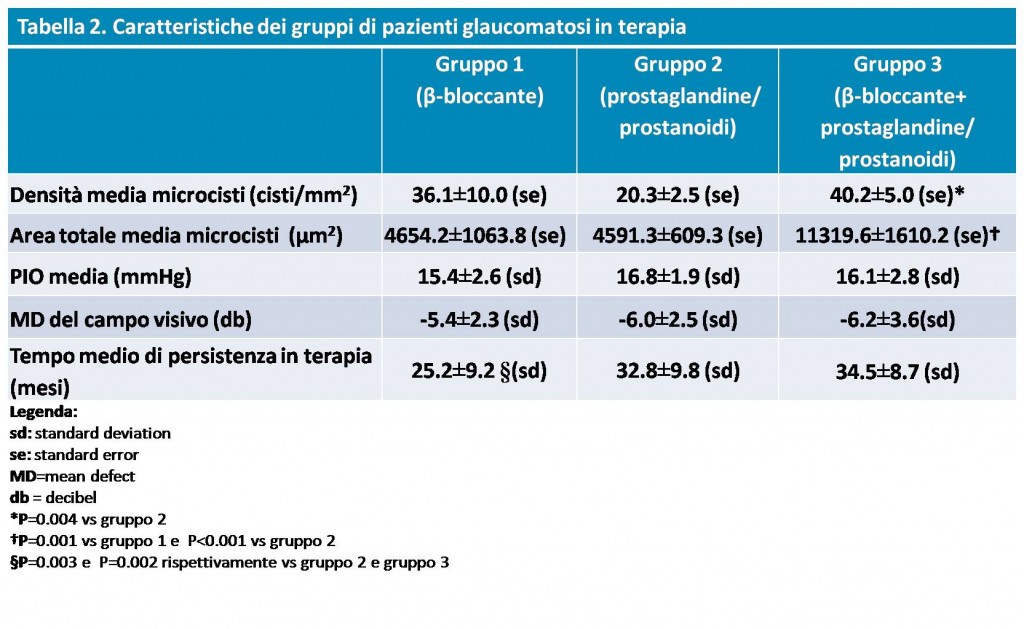
Significant differences were found, however, between the 3 subgroups of glaucomatous patients undergoing treatment, with higher mean microcystic density values in Group 3 than in Group 2 and higher mean total area values in Group 3 than in Group 1.
Conclusions
Some important conclusions can be drawn from the results obtained, which in several respects constitute new concepts in the pathophysiology of ocular hydrodynamics and glaucomatous disease. In fact, the presence of intra-epithelial conjunctival microcysts in eyes with untreated ocular hypertension is a clear sign of the activation of alternative outflow pathways under hyperbaric conditions: in the presence of high IOP and probable saturation of the canonical trabecular outflow pathways, the aqueous humour ultimately attempts to leave the eyeball by trans-scleral pathways, permeating the sclera and conjunctiva in succession. The same happens, without any substantial differences, in glaucomatous patients undergoing treatment, testifying to the fact that trans-scleral outflow is activated very early in the process.
Analysing the ocular pressure factor, given the absence of a significant correlation between IOP and microcyst density-area in both OH and POAG patients, it appears that the elevated IOP element constitutes a fundamental moment in the activation of the process, although it is not its sole determinant. This hypothesis could in fact be confirmed by the evidence that intra-ocular pressure does not appear to be necessary in the drive to maintain filtration, given the persistence of trans-scleral outflow despite pharmacological IOP control in glaucomatous patients.
On the basis of the initial considerations and in the light of the results obtained in the present study, we can ultimately hypothesise that under hyperbaric conditions hydrodynamic compensation mechanisms are activated that exploit scleral hydraulic conductivity, by virtue of which the closed system of the eyeball would try to reduce the internal stress forces (IOP-dependent) by discharging aqueous humour beyond the conjunctiva. Moreover, we are certainly not dealing with artefacts due to the active ingredients or preservatives in anti-glaucomatous drugs, given the presence of microcystic structures even in ocular hypertensives not undergoing therapy. The differences between the subgroups of glaucomatous patients on drug therapy are, however, difficult to explain with the data acquired so far and must be verified with further studies. In conclusion, the trans-scleral outflow pathways demonstrated now also in vivo, show themselves to be fundamental and primary in the compensation mechanisms that the eye can draw on under hyperbaric conditions, where trabecular outflow is reduced. The first consequence is that the conjunctiva, finally definable as the terminal of this pathway, constitutes a new target structure, easily accessible for diagnostics, where the changes induced by glaucomatous disease can be researched and studied. Analysing this in therapeutic terms, having in the near future drugs that can exploit and increase this outflow pathway, could certainly enable better and more effective management of IOP.
Leonardo Mastropasqua,
Marco Ciancaglini,
Luca Agnifili
Ophthalmology Clinic - Centre of Excellence in Ophthalmology University G. d'Annunzio Chieti-Pescara
Bibliography
1. Weinreb RN, Toris CB, Gabelt BT, et al. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002; 47(suppl 1):S53-S64.
2. Inomata H, Bill A. Exit sites of uveoscleral flow of aqueous humor in cynomolgus monkey eyes. Exp Eye Res. 1977;25:113-118.
3. Lindsey JD, Weinreb RN. Identification of the Mouse Uveoscleral Outflow Pathway Using Fluorescent Dextran. Invest Ophthalmol Vis Sci. 2002;43:2201-2205.
4. Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv Drug Deliv Rev. 2005;57:2063-2079.
5. Ambati J, Gragoudas ES, Miller JW, et al. Transscleral delivery of bioactive protein to the choroid and retina. Invest Ophthalmol Vis Sci. 2000;41:1186-1191.
6. Olsen TW, Aaberg SY, Geroski DH, Edelhauser HF. Human sclera: thickness and surface area. Am J Ophthalmol. 1998;125:237-241.
7. Labbé A, Dupas B, Hamard P, Baudouin C. In vivo confocal microscopy study of blebs after filtering surgery. Ophthalmology 2005; 112:1979.
8. Ciancaglini M, Carpineto P, Agnifili L, Nubile M, Lanzini M, Fasanella V, Mastropasqua L. Filtering Bleb Functionality: a Clinical, Anterior Segment Optical Coherence Tomography and In Vivo Confocal Microscopy Study. J Glaucoma. 2008. Jun-Jul;17(4):308-17.
9. Ciancaglini M, Carpineto P, Agnifili L, Nubile M, Fasanella V, Mastropasqua L. Conjunctival modifications in ocular hypertension and primary open angle glaucoma: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2008. Jul;49(7):3042-8. Epub 2008 Mar 3.
Dr. Carmelo Chines
Direttore responsabile




