The myopia is a of the main causes of visual impairment in the world. Over the past two decades, this disease has had an almost pandemic prevalence, registering around 1.406 billion cases (22.9% of the global population) in 2000, of which 2.7% had a high degree of myopia (-6.00 diopters or worse). It is expected that the World prevalence of myopia will increase to 49.8% by 2050.
Despite the possibility of correction, myopia is still a pathology of concern, as it represents the dominant risk factor for certain eye diseases such as myopic maculopathy, retinal detachment, glaucoma and cataracts. For each of these conditions, the risk increases as the degree of myopia increases. Therefore, there is an urgent need to define the pathogenesis behind the onset of the disease and to develop effective and safe therapeutic interventions for the prevention of associated complications.
Nearsightedness is caused by an excessive axial elongation of the eye that causes the retina to lie behind the focal plane, this change is accompanied by the thinning of the sclera, maintaining ocular shape and integrity. Various studies have investigated the mechanisms underlying myopia-related thinning and weakening of the sclera, among them some have demonstrated a role of the remodelling of the scleral extracellular matrix (ECM) related to the decelerated synthesis and accelerated degradation of ECM components. For example, during the onset of myopia, the turnover of type I collagen (the main component of scleral CME) increases due to down-regulation of its synthesis and an increase in its degradation, leading to a change in the scleral ECM. Despite these insights and numerous experimental models, the physiological signals that trigger these changes still remain unknown. Some genetic and pharmacological studies have hypothesised the involvement of several molecular signals in the development of myopia, including retinoic acid, acetylcholine, retinal dopamine, TGF-? scleral receptor-mediated signalling and A2A receptors for adenosine. In all cases the nature of the mediators of communication between retina and sclera remains unknown. Molecular analysis of the mechanisms underlying scleral remodelling is not straightforward, there is in fact no method to deal with the cellular heterogeneity of scleral tissues. The mammalian sclera is composed of a single fibrous layer of connective tissue containing fibroblasts and myofibroblasts. The former secrete type I collagen, which is the main component of collagen fibres and other ECM components, while myofibroblasts are contractile cells derived from the gradual trans-differentiation of fibroblasts. Isolating and analysing each of these different scleral cell types, in order to identify possible signal mediators that activate scleral remodelling of the ECM and the development of myopia, is experimentally difficult.
In this study, the authors used the single-cell RNA sequencing (scRNA-seq) for identify the distinct expression profiles of sclera cell populations in order to shed light on cellular phenotypic changes (i.e. fibroblast/myofibroblast trans-differentiation) and ECM alterations during the pathogenesis of myopia.
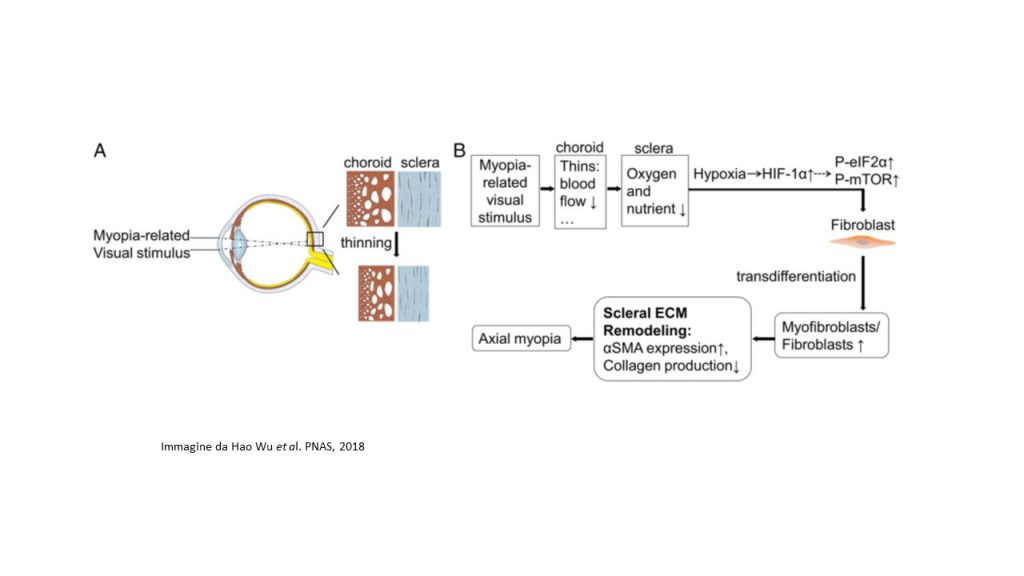 The study recently published in PNAS, pointed out that, in the myopic sclera of the mouse models, the signals of hypoxia, namely the pathways eIF2 (Eukaryotic Initiation Factor 2) and mTOR (mammalian target of rapamycin), were activated. The authors, with multiple lines of experimental evidence establish that scleral hypoxia is a critical factor that contributes to the development of myopia. Firstly, scRNA-seq analysis revealed that the development of myopia is associated with the trans-differentiation of fibroblasts into myofibroblasts, whose main functional differences arise from the activation of hypoxia-related pathways (eIF2 and mTOR signalling pathways). Many genes involved in hypoxia-inducible factor HIF-1 signalling pathway? (Hypoxia-inducible factor 1-alpha) were also, associated with risk genes involved in human myopia or pathological myopia.
The study recently published in PNAS, pointed out that, in the myopic sclera of the mouse models, the signals of hypoxia, namely the pathways eIF2 (Eukaryotic Initiation Factor 2) and mTOR (mammalian target of rapamycin), were activated. The authors, with multiple lines of experimental evidence establish that scleral hypoxia is a critical factor that contributes to the development of myopia. Firstly, scRNA-seq analysis revealed that the development of myopia is associated with the trans-differentiation of fibroblasts into myofibroblasts, whose main functional differences arise from the activation of hypoxia-related pathways (eIF2 and mTOR signalling pathways). Many genes involved in hypoxia-inducible factor HIF-1 signalling pathway? (Hypoxia-inducible factor 1-alpha) were also, associated with risk genes involved in human myopia or pathological myopia.
The research team also pointed out that exposure to hypoxia (5% O2) promoted myofibroblast trans-differentiation with down-regulation of type I collagen in human scleral fibroblasts. Another important finding of the study is that, in guinea pigs, the use of anti-hypoxia compounds, such as salidroside and formononetin, down-regulated HIF-1?, as well as the phosphorylation levels of eIF2? and mTOR, thus slowing down the progression of myopia experimental, without altering normal ocular growth. Furthermore, inhibition of phosphorylation of eIF2? suppressed myopia experimentally induced, while the phosphorylation of mTOR induced myopia in healthy mouse models.
Taken together, the results of this study defined an essential role of hypoxia in the remodelling of the scleral ECM and the development of myopia. The identification of scleral hypoxia in myopia contributes not only to understanding the mechanisms of myopia development, but also suggests a viable therapeutic approach to control the progression of myopia in humans.
Source
Scleral hypoxia is a target for myopia control. Hao Wu and tol. Proceedings of the National Academy of Sciences (PNAS). Jul 2018.
Dr. Carmelo Chines
Direttore responsabile
