The new therapeutic potential of stem cells in ophthalmology.
Recent advances in regenerative medicine, in particular therapies based on the use of autologous adult stem cell cultures, and the first recently proposed clinical trials with pluripotent stem cells, have generated enthusiasm and much effort to explore the new therapeutic potential of both adult and pluripotent stem cells.
In recent decades, developmental biology has elucidated many cellular and molecular mechanisms that regulate stem cell-dependent tissue homeostasis, making us aware of the molecular basis of many diseases. Many human tissues and organs possess the ability to self-renew and repair acute and chronic injuries. These processes are based on the presence of specific stem cells, which generate progenitors (often referred to as transiently amplifying cells), which generate terminally differentiated cells. Typical examples of these transitions are haematopoietic stem cells, which give rise to all blood cells through myeloid and lymphoid progenitors, or epidermal stem cells, which give rise to the epidermis, hair follicle and sebaceous glands; the mesenchymal stem cells, which derive from the bone marrow, are capable of generating all the tissues found in the bone segment (bone, cartilage, adipocytes, fibroblasts and the stroma that supports haematopoiesis), better defined as skeletal stem cells.
of the corneal epithelium, have been shown to completely restore a severely damaged corneal epithelium and allow recovery of vision in patients with corneal destruction caused by chemical burns. The stem cells are isolated by enzymatic treatment from a 1-2 mm biopsy of limbus, the thin zone between the cornea and conjunctiva. The limbus is the only corneal zone with papillae-like invaginations, called Vogt Palisades e limbal epithelial cryptscontaining many small basal cells that lack keratin 3, which is specific for corneal differentiation. This limbal basal layer contains cells that slowly complete the cell cycle and form holoclones, whereas central corneal cells do not. Many molecular markers have been proposed to identify corneal stem cells, however, correlation with the ability to regenerate the cornea in the long term has only been demonstrated for a few of them.
Shortly after the first report of successful clinical application with cultured limbal stem cells, several dozen similar protocols were proposed with related clinical applications. Investigations into alternative methods and pathologies eligible for treatment have contributed to increasing our knowledge in this field, while questions related to the identification of the causes of variability related to the reagents used for tissue reconstruction, the selection of patients included in the treatment, the drugs used in parallel, surgical and post-operative management, and the implications of these on the success rate, safety and reproducibility of clinical outcomes have increased.
The cultural separation between different scientific fields makes it difficult to establish multidisciplinary criteria that are, however, necessary for optimal translational research. In this article, an attempt is made to propose some criteria (specifically related to both clinical and biological parameters) for translational medicine with cultivated limbal stem cells.
We also offer an extensive bibliography to serve as a guide for those who wish to learn more about this field of research.
Patient selection
The limbal stem cell pool of the corneal epithelium decreases due to hereditary or acquired damage, leading to partial or total limbal stem cell deficiency (LSCD). This definition refers to a heterogeneous group of pathologies that have a deleterious effect on corneal integrity and wound closure. As a consequence of stem cell depletion, invasion of the peripheral and central cornea by conjunctival epithelium occurs. This tissue delocalisation induces neovascularisation of a normally avascular ocular area and induces corneal opacification, resulting in a severe drop in visual acuity or blindness. Conjunctival invasion can be prevented or prevented by the relocalisation of corneal stem cells obtained by transplantation of flaps generated from the culture of autologous limbal cells taken from the undamaged or less damaged eye.
[caption id="attachment_1536" align="alignright" width="178"]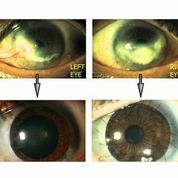 Ph. 2. Bilateral LSD before and after treatment (courtesy of Dr. Paolo Rama).[/caption].
Ph. 2. Bilateral LSD before and after treatment (courtesy of Dr. Paolo Rama).[/caption].
Appropriate selection and preparation of the 'recipient bed' are of great importance for a successful clinical outcome of limbal cultures. Failures may occur due to the severity of the damage, the degree of inflammation and post-operative complications. Chemical burns can damage the eyelids, conjunctiva and lacrimal apparatus. In the course of such damage, the ocular surface is chronically inflamed and the resulting alteration of the microenvironment can impede the take-over of cultured stem cells. In the case of extensive damage of the entire ocular surface, reconstruction of the conjunctiva, which allows movement of the eyeball and physiological distribution of the tear film, must be achieved prior to cell transplantation. In the absence of systemic or genetic negative stimuli, transplantation of autologous limbal cell cultures can itself partially or fully restore the macro- or microenvironment. This may explain why, in ocular surfaces severely damaged by chemical burns, the second transplantation of cultured epithelium produces a higher degree of success than the first transplantation. The first transplantation of cultured cells probably 'normalises the environment' through the production of matrix molecules such as laminin 5, proteoglycans and collagen, the paracrine and autocrine secretion of a balanced amount of growth factors, such as TGF alpha, interleukins, PDGF, Insulin like Growth Factor 1, TGF beta, Nerve Growth Factor, basic Fibroblasts Growth Factor, and epithelial-mesenchymal 'cross-talk'. Despite many years of proven clinical success, we still have no idea how many stem cells can take root on the recipient bed of the lesion. Moreover, ocular surface pathologies attributed to limbal stem cell deficiency may have a variety of aetiologies, but not all of them have been shown to be associated with total limbal stem cell depletion. In fact, they could be characterised by a macro- and micro-environment that is not permissive for appropriate proliferation and differentiation of existing stem cells. Varieties of limbal deficits could provide 'behavioural instructions' to transplanted cells, which guide different cellular responses during or after rooting.
Biological Parameters
Several methods have been proposed for the culture of limbal stem cells. The composition of the culture medium plays an important role in preserving limbal stem cells, and different mixtures of materials and reagents for culture have been proposed. Among the various proposals, it is unclear when Good Manufacturing Practice or GMP (which has come into force in Europe for these products) has been applied to biological reagents intended for the manufacture of advanced therapies (cultured tissues).
[caption id="attachment_1537" align="alignleft" width="178"]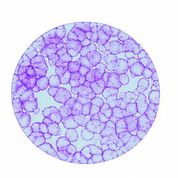 Ph. 3. Colonies of epithelial cells obtained from a stem cell (courtesy of the 'Stefano Ferrari' Centre for Regenerative Medicine).[/caption].
Ph. 3. Colonies of epithelial cells obtained from a stem cell (courtesy of the 'Stefano Ferrari' Centre for Regenerative Medicine).[/caption].
In fact, even the details of the criteria used for the selection of appropriate materials and reagents have not been regularly described and it has never been explained how these materials were considered suitable for maintaining stem cells in culture. In the absence of these data, it becomes difficult to assess the clinical success rate or safety of each developed culture system, and whether the differences are related to the culture technique or the selection of individual reagent batches. Obviously, the comparison of different culture conditions cannot show differences if the stem cells were not maintained in any of the conditions.
In past years, various studies have proposed different combinations of growth factors and hormones in the culture medium. These media can induce stimulation of different metabolic pathways with a variety of cellular responses both in culture and after transplantation. To produce clinical success, a corneal cell culture must contain sufficient numbers of keratinocyte stem cells, which are essential for long-term corneal renewal, rather than a fully stratified epithelium in culture. Clinical results obtained with different techniques may help identify key information on the appropriateness of the culture process.
The problem of xenogenic components
[caption id="attachment_1538" align="alignright" width="263"]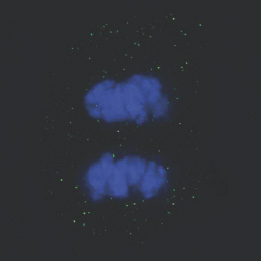 Ph. 5. A limbal cell in mitosis (courtesy of the 'Stefano Ferrari' Centre for Regenerative Medicine)[/caption].
Ph. 5. A limbal cell in mitosis (courtesy of the 'Stefano Ferrari' Centre for Regenerative Medicine)[/caption].
Culture media often contain animal-derived materials, such as bovine serum. Foetal serum is used in this, as well as in other cell culture models. Some authors have proposed the use of autologous human serum, with the intention of preventing putative xenogenic contaminants, to replace foetal bovine serum. It is, however, necessary to consider the variable content of hormones and growth factors, due to the different genetic characteristics of each individual, which would make the cultures non-reproducible, as they would grow in culture media with a different composition for each patient. These differences may have an impact on the maintenance of stem cells, and would reduce the reliability of culture controls by making it impossible to define specific, well-defined criteria for assessing the quality of cell culture. Frequently used foetal bovine serum is derived from mixtures of different sera, so individual variability in content is minimised; certainly the foetal serum used must be analysed extensively for pathogen absence in accordance with current health regulations. Equivalent use of mixtures of human sera could obviate individual variability, however, the risk of contamination by viruses, infectious or viral agents and prions also exists for reagents of human origin and, especially in the absence of species barriers, anything can be transmitted.
[caption id="attachment_1539" align="alignleft" width="178"]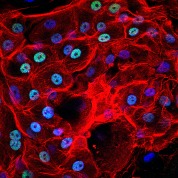 Ph. 5. Corneal cells seen under a fluorescence microscope (courtesy of the Centre for Regenerative Medicine 'Stefano Ferrari').[/caption]
Ph. 5. Corneal cells seen under a fluorescence microscope (courtesy of the Centre for Regenerative Medicine 'Stefano Ferrari').[/caption]
Another proposed choice is synthetic culture media, which, however, lack a huge number of physiological enhancers, some of which are known and could in future be produced by synthesis and addition. It must be considered, however, that many of these are still unknown and the purification (under GMP conditions) of all the necessary hormones and growth factors would make the cost of the media unattainable. Similar considerations apply to many other elements of culture media, often of animal origin and not yet replaceable if cellular functions are to be maintained, which must be carefully checked before use.
Biomechanical signals and surface modification
A typical strategy, used in stem cell-based therapies, is to reconstruct engineered tissues with cells grown on biomaterials that can 'mimic' the biochemical and biophysical microenvironment in vivo. For this purpose, many different materials have been proposed to cultivate the cells, such as fibrin, amniotic membrane, plastic or polymers of various kinds. The interaction between stem cells, their surrounding microenvironment and external forces (which represent the stem cells' 'niche') must be well understood before therapies can be applied clinically. Cell behaviour is not only governed by chemical signals. Tissue architecture and mechanical forces, to which they are subjected, are dominant signals that govern cellular decisions: cell-matrix and cell-cell adhesion interactions, the organisation of the cytoskeleton, and the tension forces that keep individual cells and the whole tissue in a precise shape, all represent, biological 'architecture' signals. The mechanical connections between the matrix and cytoskeleton allow cells to exert tensile forces that are transmitted to the cell nucleus, the result of which is the triggering of signals that modulate adhesion, surface diffusion, migration, proliferation and differentiation. More generally, mechano-transduction represents a combination of signals, including the juxtaposition of molecular, topographical and mechanical stimuli, present at the interaction interface. To study these interactions, surfaces exposing selected bio-functional molecular groups or specifically engineered micrometre-scale moulds were used. These tests showed that substrate hardness, surface nano-topography, micro geometry and extracellular forces, and even fluid fluxes, can all have an important influence on the regulation of the activities of different cell types. For example, in vivothe formation of skin scars activates Transforming Growth Factors and SMAD signalling, adhesion molecules such as integrins and calcium signals; or in the case of myoblasts, when laid on large strips of soft hydrogel, they decrease their proliferation and increase their fusion index.
Medication during surgery and follow-up
The management of post-traumatic or post-operative problems involves extensive use of drugs. In the case of treatment for limbal stem deficits, the impact of acute and chronic corneal toxicity of eye drop components is not adequately considered in regenerative medicine protocols. In spite of this, a large number of experimental and clinical studies have shown that prolonged use of topical drugs can induce ocular surface changes, causing ocular discomfort, tear film instability, loss of calico cells, inflammation, squamous conjunctival metaplasia, epithelial apoptosis, and subconjunctival fibrosis. Prolonged use of local anaesthetics is associated with delayed corneal re-epithelialisation after injury, altered lubrication and tear film, corneal swelling and disruption of epithelial motility. Lidocaine, one of the most commonly used anaesthetics, already at a dose of 250 mg/ml (below the clinical dose), reduces the effectiveness of normal wound healing. Vasoconstrictors, which are generally used to increase the duration of local anaesthesia, may produce a cytotoxic effect and pigment deposition, as reported in the literature for epinephrine and some commonly used anti-glaucoma drugs.
Depending on dosage and mode of administration, corticosteroids, which are widely used, both systemically and topically, in the treatment of ocular inflammatory conditions, have side effects that include delaying or preventing the closure of corneal lesions. Non-steroidal anti-inflammatory drugs inhibit prostaglandin synthesis and exert an equivalent anti-inflammatory effect. These drugs are increasingly used for their concomitant analgesic effect and efficacy in ocular allergic diseases.
Comparative analysis of corneal toxicity revealed cell damage, altered cell viability, proliferation and migration even after short-term exposure of corneal cells to certain non-steroidal anti-inflammatory drugs. Other commonly used drugs, such as prostaglandin analogues, anti-allergy drops or multipurpose solutions (especially in the presence of preservatives) showed similar toxic effects. In particular, the scientific data suggest an interpretation of irreversible damage of the epithelial proliferative compartment, which includes stem cells; effects on cell viability (with a decrease up to 40%), proliferation, maintenance of corneal thickness, endothelial permeability, DNA integrity and lubrication have been described, due to common preservatives such as benzalkonium chloride (BAC), especially in the case of repeated administration or persistence of these drugs on damaged ocular surfaces.
It is clear that all these toxic effects are amplified when drugs are used prior to the full rooting of a cultured cell population.
In conclusion, no drug appears to be free of toxicity of some kind, so the selection of drugs on applied cell cultures and the harmonisation of dosages and administration protocols must be carefully considered to increase the benefit/risk ratio.
Conclusions
Rapid advances in stem cell research have raised the interest of governments, the media and, of course, patients. Their clinical success depends on factors unique to cell therapies, which include manufacturing procedures, standardisation of clinical and pharmacological protocols, and safety regulation. As already discussed, successful clinical application of any cell therapy protocol requires optimisation of the culture method (especially when stem cells are required) and surgical procedures, control of the microenvironment in which the cells are to take root, and appropriate pharmacological support.
This scenario is further complicated by new regulations on clinical applications of cells and tissues. As with many traditional drugs, cell cultures for clinical application must be obtained in accordance with current Good Manufacturing Practice (GMP) standards, but cell cultures are inherently more complex and less well controlled than a small molecule. Due to their biological nature, living cell products cannot be fully defined with the same methods as traditional drugs. The choice to apply the rules of medicinal products to cell cultures, without specific adaptations for this field, is not entirely acceptable. Indeed, this choice creates problems for scientists and regulators alike. Researchers from both academia and industry can hardly cope with the new European rules on ATMPs (Advanced Therapy Medicinal Products) (EC regulation n°1394). Similar, but not identical, regulation has been applied in the United States and other industrialised countries.
Products based on living cells present many additional challenges especially in today's highly regulated healthcare environment, especially considering that the web of regulations was designed for chemical-type manufacturing in the last century. In addition, there is not much harmonisation between different regulatory authorities, which greatly increases the problems in manufacturing and clinical trials. The international conference for the harmonisation of technical requirements for the registration of pharmaceuticals for human use has only produced agreements on specific topics, such as viral safety and a few others.
Regulation is intended to increase safety, and thus protect patients, and is unquestionable; however, for products based on living cells, it is often achieved at the expense of efficacy, due to a lack of sector-specific adjustments. Every therapy is based on a benefit/risk ratio, so reducing the efficacy of a cell culture below a certain threshold value will generate a safer but useless biological product. Therapies based on the use of living cells, are more complex and inherently less controlled than the synthesis of molecules, due to their biological nature, making this product not fully but only partially defined, according to 'classical' regulations, which require expansion for these reasons. Finally, many patient-specific autologous cell products are 'freshly administered', so release assays cannot all be completed prior to administration, leading us to the idea that the real product is the manufacturing process, and the reliability of manufacture should be periodically assessed through comparison with specific reference cells, determining the limits for the controls that are obtained during tissue manufacture. These limits will be wider for tissue manufacture from different individuals, due to differences in age, sex, lifestyle, and individual genetic characteristics, but should be tighter for large-scale donor treatments.
An understanding of the prospects for regenerative medicine will provide insight into the likely future shape of new therapies, their development timeframes, as well as the planning of the infrastructure required to facilitate its expeditious deployment by academies and the new pharmaceutical industry worldwide, with maximum guarantees for patients.
Graziella Pellegrini
E-mail: graziella.pellegrini@unimore.it
Prof. Graziella Pellegrini is Professor of Biology at the University of Modena and Reggio Emilia and Cell Therapy Coordinator at the 'Stefano Ferrari' Centre for Regenerative Medicine, Prof. Pellegrini has devoted much of her scientific activity to translational medicine and the development of clinical applications of cultured stem cells.
Together with Prof. Michele De Luca, he developed the first treatment with human limbal stem cells for patients with severe corneal burns.
He is one of the founding members of the IOSS (International Ocular Surface Society).
Bibliographic references
1. Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol.13:497-505.
2. Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639-648.
3. Tedesco FS, Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol. 2012;25:597-603.
4. Seita J, Weissman IL. Hematopoietic stem cells: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640-653.
5. Claudinot S, Nicolas M, Oshima H, et al. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci U S A. 2005;102:14677-14682.
6. Blanpain C, Horsley V, Fuchs E. Epithelial Stem Cells: Turning over New Leaves. Cell .. 2007;128:445-458.
7. Jungebluth P, Alici E, Baiguera S, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. 2011;378:1997-2004.
8. White P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35-42.
9. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331-343.
10. Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979;76:5665-5668.
11. Gallico GG, 3rd, O'Connor NE, Compton CC, et al. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448-451.
12. De Luca M, Albanese E, Bondanza S, et al. Multicentre experience in the treatment of burns with autologous and allogenic cultured epithelium, fresh or preserved in a frozen state. Burns. 1989;15:303-309.
13. Pellegrini G, Ranno R, Stracuzzi G, et al. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation. 1999;68:868-879.
14. Ronfard V, Rives JM, Neveux Y, et al. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation. 2000;70:1588-1598.
15. Mavilio F, Pellegrini G, Ferrari S, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397-1402.
16. Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769-782.
17. Schwab IR. Cultured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc. 1999;97:891-986.
18. Tsai RJ, Li L, Chen J. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells(1). Am J Ophthalmol. 2000;130:543.
19. Rama P, Bonini S, Lambiase A, et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72:1478-1485.
20. Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147-155.
21. Dua HS, Shanmuganathan VA, Powell-Richards AO, et al. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89:529-532.
22. Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201-209.
23. Di Iorio E, Barbaro V, Ruzza A, et al. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci U S A. 2005;102:9523-9528.
24. Priya CG, Prasad T, Prajna NV, et al. Identification of Human Corneal Epithelial Stem Cells on the Basis of High ABCG2 Expression Combined With a LargeN/C Ratio. Microsc Res Tech.76:242-248.
25. Kubota M, Shimmura S, Miyashita H, et al. The anti-oxidative role of ABCG2 in corneal epithelial cells. Invest Ophthalmol Vis Sci.51:5617-5622.
26. Meyer-Blazejewska EA, Kruse FE, Bitterer K, et al. Preservation of the limbal stem cell phenotype by appropriate culture techniques. Invest Ophthalmol Vis Sci.51:765-774.
27. Santos MS, Gomes JA, Hofling-Lima AL, et al. Survival analysis of conjunctival limbal grafts and amniotic membrane transplantation in eyes with total limbal stem cell deficiency. Am J Ophthalmol. 2005;140:223-230.
28. Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet .. 1997;349:990-993.
29. Li DQ, Lee SB, Tseng SC. Differential expression and regulation of TGF-beta1, TGF-beta2, TGF-beta3, TGF-betaRI, TGF-betaRII and TGF-betaRIII in cultured human corneal, limbal, and conjunctival fibroblasts. Curr Eye Res. 1999;19:154-161.
30. Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61-79.
31. Touhami A, Grueterich M, Tseng SC. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci. 2002;43:987-994.
32. Dell S, Peters S, Muther P, et al. The role of PDGF receptor inhibitors and PI3-kinase signaling in the pathogenesis of corneal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:1928-1937.
33. Lambiase A, Aloe L, Mantelli F, et al. Capsaicin-induced corneal sensory denervation and healing impairment are reversed by NGF treatment. Invest Ophthalmol Vis Sci. 2012;53:8280-8287.
34. Qi H, Li DQ, Shine HD, et al. Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells. Exp Eye Res. 2008;86:34-40.
35. Sakimoto T, Sawa M. Metalloproteinases in corneal diseases: degradation and processing. Cornea. 2012;31 Suppl 1:S50-56.
36. Jordan T, Hanson I, Zaletayev D, et al. The human PAX6 gene is mutated in two patients with aniridia. Nat Genet. 1992;1:328-332.
37. Mishra R, Gorlov IP, Chao LY, et al. PAX6, paired domain influences sequence recognition by the homeodomain. J Biol Chem. 2002;277:49488-49494.
38. Tohyama M, Watanabe H, Murakami S, et al. Possible involvement of CD14+ CD16+ monocyte lineage cells in the epidermal damage of Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2012;166:322-330.
39. De Luca M, Pellegrini G, Green H. Regeneration of squamous epithelia from stem cells of cultured grafts. Regenerative Med. 2006;1:45-57.
40. Guerra L, Capurro S, Melchi F, et al. Treatment of "stable" vitiligo by timedsurgery and transplantation of cultured epidermal autografts. Arch Dermatol. 2000;136:1380-1389.
41. Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95:1525-1529.
42. Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci U S A. 1987;84:2302-2306.
43. Rochat A, Kobayashi K, Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell .. 1994;76:1063-1073.
44. Shimazaki J, Higa K, Morito F, et al. Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. Am J Ophthalmol. 2007;143:945-953.
45. Satake Y, Higa K, Tsubota K, et al. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology. 2011;118:1524-1530.
46. Shahdadfar A, Haug K, Pathak M, et al. Ex vivo expanded autologous limbal epithelial cells on amniotic membrane using a culture medium with human serum as single supplement. Exp Eye Res.97:1-9.
47. Stacey GN, Cobo F, Nieto A, et al. The development of 'feeder' cells for the preparation of clinical grade hES cell lines: challenges and solutions. J Biotechnol. 2006;125:583-588.
48. Liu ZZ, Chen P, Lu ZD, et al. Enrichment of breast cancer stem cells using a keratinocyte serum-free medium. Chin Med J (Engl). 2011;124:2934-2936.
49. Kormos B, Belso N, Bebes A, et al. In vitro dedifferentiation of melanocytes from adult epidermis. PLoS One. 2011;6:e17197.
50. Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science .. 2005;310:1139-1143.
51. Green H. The birth of therapy with cultured cells. Bioessays. 2008;30:897-903.
52. Carrier P, Deschambeault A, Audet C, et al. Impact of cell source on human cornea reconstructed by tissue engineering. Invest Ophthalmol Vis Sci. 2009;50:2645-2652.
53. Sharma SM, Fuchsluger T, Ahmad S, et al. Comparative analysis of human-derived feeder layers with 3T3 fibroblasts for the ex vivo expansion of human limbal and oral epithelium. Stem Cell Rev. 2012;8:696-705.
54. Oie Y, Hayashi R, Takagi R, et al. A novel method of culturing human oral mucosal epithelial cell sheets using post-mitotic human dermal fibroblast feeder cells and modified keratinocyte culture medium for ocular surface reconstruction. Br J Ophthalmol. 2010;94:1244-1250.
55. Paliwal P, Sharma A, Tandon R, et al. TGFBI mutation screening and genotype-phenotype correlation in north Indian patients with corneal dystrophies. Mol Vis. 2010;16:1429-1438.
56. West MD. The cellular and molecular biology of skin ageing. Arch Dermatol. 1994;130:87-95.
57. Hultman CS, Brinson GM, Siltharm S, et al. Allogeneic fibroblasts used to grow cultured epidermal autografts persist in vivo and sensitize the graft recipient for accelerated second-set rejection. J Trauma. 1996;41:51-58; discussion 58-60.
58. Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591-600.
59. Shi ZD, Tarbell JM. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann Biomed Eng. 2011;39:1608-1619.
60. Weinbaum S, Guo P, You L. A new view of mechanotransduction and strain amplification in cells with microvilli and cell processes. Biorheology. 2001;38:119-142.
61. Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature .. 2011;474:179-183.
62. Zatti S, Zoso A, Serena E, et al. Micropatterning topology on soft substrates affects myoblast proliferation and differentiation. Langmuir. 2012;28:2718-2726.
63. Chowdhury F, Li Y, Poh YC, et al. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS One. 2010;5:e15655.
64. Huang C, Akaishi S, Ogawa R. Mechanosignaling pathways in cutaneous scarring. Arch Dermatol Res. 2012;304:589-597.
65. Ramselaar JA, Boot JP, van Haeringen NJ, et al. Corneal epithelial permeability after instillation of ophthalmic solutions containing local anaesthetics and preservatives. Curr Eye Res. 1988;7:947-950.
66. Burstein NL. Corneal cytotoxicity of topically applied drugs, vehicles and preservatives. Surv Ophthalmol. 1980;25:15-30.
67. Rosenwasser GO. Complications of topical ocular anaesthetics. Int Ophthalmol Clin. 1989;29:153-158.
68. Bisla K, Tanelian DL. Concentration-dependent effects of lidocaine on corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1992;33:3029-3033.
69. Krejci L, Harrison R. Antiglaucoma drug effects on corneal epithelium. A comparative study in tissue culture. Arch Ophthalmol. 1970;84:766-769.
70. Qu M, Wang Y, Yang L, et al. Different cellular effects of four anti-inflammatory eye drops on human corneal epithelial cells: independent in active components. Mol Vis. 2011;17:3147-3155.
71. Nguyen DQ, Srinivasan S, Hiscott P, et al. Thimerosal-induced limbal stem cell failure: report of a case and review of the literature. Eye Contact Lens. 2007;33:196-198.
72. Zhou Y, Liu Q, Zhou T, et al. Modulation of the canonical Wnt pathway by Benzalkonium Chloride in corneal epithelium. Exp Eye Res. 2011;93:355-362.
73. Chen W, Li Z, Hu J, et al. Corneal alternations induced by topical application of benzalkonium chloride in rabbit. PLoS One. 2011;6:e26103.
74. Ye J, Wu H, Zhang H, et al. Role of benzalkonium chloride in DNA strand breaks in human corneal epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2011;249:1681-1687.
75. Mason C, Dunnill P. Translational regenerative medicine research: essential to discovery and outcome. Regen Med. 2007;2:227-229.
Dr. Carmelo Chines
Direttore responsabile






