Towards the development of an effective and safe eye drops for the treatment of Acanthamoeba keratitis.
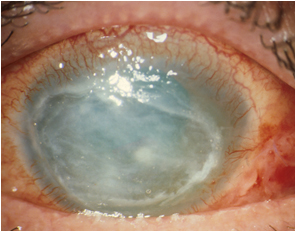 Once again this year, the ODAK Consortium, led by SIFI, was present at the ARVO (Association for Research in Vision and Ophthalmology) Conference, Baltimore 7-11 May 2017, with two Posters describing the latest results on the pharmaceutical development of an eye drop containing PHMB 0.08%, intended for the treatment of Acanthamoeba keratitis. This is a rare condition that can lead to blindness if not adequately treated. To date, there is no drug approved for the treatment of Acanthamoeba keratitis.
Once again this year, the ODAK Consortium, led by SIFI, was present at the ARVO (Association for Research in Vision and Ophthalmology) Conference, Baltimore 7-11 May 2017, with two Posters describing the latest results on the pharmaceutical development of an eye drop containing PHMB 0.08%, intended for the treatment of Acanthamoeba keratitis. This is a rare condition that can lead to blindness if not adequately treated. To date, there is no drug approved for the treatment of Acanthamoeba keratitis.
 In the poster entitled "A 26-week repeated-dose toxicity study of PHMB 0.08% ophthalmic solution in rabbits" demonstrates the safety of eye drops in rabbits after a 26-week administration period. In the other entitled "Ocular safety and tolerability of high dose PHMB (Polyhexanide) in healthy volunteers"we report the excellent safety results of PHMB eye drops at the three different concentrations tested (0.08%; 0.06%; 0.04%) in the 90 healthy volunteers enrolled in the Phase I clinical trial.
In the poster entitled "A 26-week repeated-dose toxicity study of PHMB 0.08% ophthalmic solution in rabbits" demonstrates the safety of eye drops in rabbits after a 26-week administration period. In the other entitled "Ocular safety and tolerability of high dose PHMB (Polyhexanide) in healthy volunteers"we report the excellent safety results of PHMB eye drops at the three different concentrations tested (0.08%; 0.06%; 0.04%) in the 90 healthy volunteers enrolled in the Phase I clinical trial.
The results obtained in the two studies presented at ARVO support, with sufficient evidence, the continuation of the development of PHMB 0.08% eye drops towards phase III clinical trials in subjects with Acanthamoeba keratitis.
Dr. Carmelo Chines
Direttore responsabile
