Idiopathic subretinal neovascularisations
In subjects under 50 years of age, the finding of a sub-retinal neovascular membrane (CNV) should promote investigations to rule out associated causes, first pathological myopia, then angioid striae, then posterior segment inflammation (post-inflammatory CNV) (1), or traumatic choroid rupture, iatrogenic (2), optic nerve aplasia (3), choroid osteoma (4), choroidal nevus (5-6), radiation retinopathy (7). Where all these causes have been ruled out, the diagnosis of idiopathic CNV is made. Idiopathic CNVs have a natural history with a benign course, tend to self-limit and regress spontaneously, irrespective of lesion site, with a useful functional residual in most cases (75% ? 20/60), and rare occurrence of recurrence (8-9).
An indication of spontaneous regression and a positive prognostic factor appears to be the presence of a hypofluorescent borderline in ICGA, which is not always visible on ophthalmoscopy and FAG, corresponding to a multi-layered proliferation of the EPR, a sign of an involutional process demonstrated in the animal model and in humans in pseudohistoplasmosis CNVs (10).
Idiopathic CNVs are classified as type 2 according to Gasswith growth above the retinal pigment epithelium: in young subjects the close anatomical adhesion between EPR and Bruch's membrane could explain this growth pattern, and the integrity of the EPR could account for the prognostic evolution better than in other forms of CNV (9, 11).
The ICGA also shows focal areas of distant vascular hyper-permeability independent of the CNV similar to those observed in central serous chorio-retinopathy, and suggests a predisposing subclinical inflammatory condition also in these forms with an etiopathogenesis that is still discussed. The OCT confirms the position above the EPR with integrity of the hyperreflective band relative to the EPR itself and shows two main patterns of behaviour: a 'protruding' appearance, associated with elevation of the macular profile, and a 'fusiform' appearance in which the hyperreflectivity of the EPR-Bruch complex is confused with that of the lesion itself. The former refers to the activity phase, while involution is indicated by the latter (12-13).
The discussed inflammatory origin of juvenile idiopathic forms finds strength in the clinical response to the steroid therapyeither by os or by the subtenonial or intravitreal route (14).
For extra-foveal forms, the choice of direct argon or krypton photocoagulation remains possible, but with no data showing a better final functional result than natural history (9).
[caption id="" align="alignleft" width="150"] Ph. 1 - idiopathic CNV pre PDT[/caption]
Ph. 1 - idiopathic CNV pre PDT[/caption]
The photodynamic therapy (PDT) in idiopathic CNV has shown efficacy in stabilising and improving visus in over 65% of patients with a low rate of treatment recurrence (mean 1.1-2.1), albeit with the associated risk of atrophic changes in the EPR in the treatment area (Figs. 1-2). The association with steroid administration resumes the rationale of the likely inflammatory aetiology, and a randomised trial showed a significantly better clinical response if PDT is combined with steroid per os (15-20).
[caption id="attachment_1948" align="alignleft" width="150"]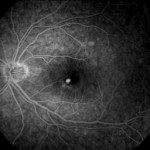 Ph. 2 - CNV Idiopathic post PDT[/caption]
Ph. 2 - CNV Idiopathic post PDT[/caption]
The therapeutic alternative is thesurgical excision which makes it possible to predict good functional results due to the type of neovascularisation (Gass type 2) and the integrity of the underlying EPR(21-22)
Finally, the introduction of the injection therapies with anti-VEGF drugs expanded the therapeutic spectrum for these forms of subretinal neovascularisation as well, with a response to single or multiple monthly administrations of bevacizumab in terms of improvement in visual acuity and absence of angiographic leakage at six months in 70-100% of cases and an improvement in visual acuity of at least two Snellen lines in 60-75% of cases. The therapeutic efficacy of bevacizumab was also observed in cases that had not responded to other therapeutic modalities (23-25).
The trans-pupillary thermotherapyin the only work highlighted in the literature, showed efficacy in improving and stabilising the visus in 81% of 21 treated patients (26).
However, we would point out that for none of the therapies listed above are there any randomised controlled efficacy studies and the indications arise from observational studies on groups of patients that are in any case limited in number and without control groups; moreover, the good prognosis of the natural history makes it obligatory in our opinion to inform the patient about the possibility of simply adopting an observation strategy.
Post-inflammatory subretinal neovascularisations
[caption id="attachment_1949" align="alignleft" width="150"]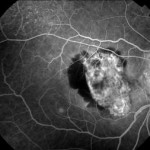 Ph. 3 - CNV post toxo[/caption].
Ph. 3 - CNV post toxo[/caption].
Subretinal neovascularisation (CNV) is a possible although rare complication of uveitis (1), both chorioretinitis and uveopapillitis, whether infectious or immunological in nature. No but sporadic data on the frequency of this complication are known, but it has been described associated with sarcoidosis and Behçet's disease (1), toxoplasmic chorioretinitis (2-3) (Figs. 3-4), perhaps the best known form together with the form complicating multifocal choroiditis (4), but also more rarely a complication of presumed histoplasmosis, multifocal choroiditis, choroiditis serpiginosa (1), Vogt-Konayagi-Harada syndrome (5) and rubella (6).
[caption id="attachment_1950" align="alignleft" width="150"]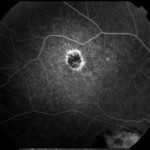 Ph. 4 - CNV post toxo[/caption].
Ph. 4 - CNV post toxo[/caption].
In the multifocal choroiditis an incidence of 22% is reported, and CNV is the leading cause of severe visual impairment in this cohort of patients (45% of cases), preceded by cystoid macular oedema and epiretinal membranes, associated with reduced visual acuity in three quarters of patients (<20/50) (1). If the duration of the disease is directly correlated with the risk of developing cystoid macular oedema, no such correlation seems to exist with the risk of developing CNV, so that subretinal neovascularisation can be considered an even early complication of the disease. Immunosuppressive therapy (azathioprine, cyclosporine, mycophenolate mofetil, etc.) seems to reduce the risk of CNV recurrence by 83%, but there are no data on the reduction of the risk of CNV occurrence itself; on the contrary, chronic use of low-dose steroids has no protective function against all posterior complications (1).
L'surgical excision is reported to be effective in cases of CNV complicated with presumptive histoplasmosis (7), multifocal choroiditis, serpiginous choroiditis, Behçet's disease (1), toxoplasmic retinochoroiditis (8). The therapeutic success of surgical excision can be explained by the condition of type 2 membranes according to Gass, similarly to what occurs in idiopathic forms, which lie above the otherwise healthy retinal pigment epithelium and with a natural cleavage plane given by the strong adhesions between EPR and Bruch's membrane (8).
However, in the face of the general abandonment of the surgical approach for CNVs, the photodynamic therapy has been used in single clinical evaluations in cases of rubella (9), Vogt-Konayagi-Harada syndrome (10), multifocal choroiditis (11-12), internal punctate choroiditis (13-15), presumptive histoplasmosis (14) and serpiginous choroiditis (15). PDT appears to be satisfactorily effective in these patients in stabilising visus and in inducing scarring of the lesion, with a mean retreatment rate of 2.6 (9-16).
However, we still consider the correctness of the indication to be a matter of debate: photodynamic therapy is per se 'inflammatory' and in applying it to these forms of neovascularisation, which recognise inflammation as the primum movens, it should at least be combined with steroid therapy in concert, either intravitreally or per os (13,17). Greater rationale for proposing the antiVEGF drug therapyVEGF is known to play a role in posterior eye inflammation and probably also in the occurrence of neovascular complications (18). Even for this therapy there are only occasional reports of treatment of small groups of patients, but no standardised treatment schemes. In a single scientific paper, bevacizumab shows therapeutic efficacy in 100% of cases in terms of clinical silence of the neovascularisation and in 90% of cases in terms of improvement of visual acuity, with the remaining 10% achieving visual stabilisation even after only one injection (19).
It is therefore appropriate to emphasise the current arbitrariness of any form of treatment for these neovascular forms in the absence of efficacy trials, which are, moreover, not easily achievable due to the limited incidence of this pathology. Rather, it would be desirable for tertiary referral structures for uveitis to plan common treatment strategies to be able to support a scientific comparison of efficacy between various protocols. In our opinion, the most correct current indication remains that of photodynamic therapy always associated with a steroid (per os, intravitreal), because the efficacy of the combination seems to be supported by the largest relative number of studies. However, the rationale for anti-VEFG drug therapy is present and convincing, although to date strengthened by only one case series study. Treatment strategies may be further changed by the expected market introduction of slow-release intravitreal cortisone devices.
Dr Maria Vadalà,
Dr. Stefano Cipolla
Department of Clinical Neuroscience, Section of Ophthalmology, University of Palermo
Bibliography idiopathic CNVs
1. Cohen SY, Laroche A, Leguen Y, Soubrane G, GJ. Etiology of choroidal neovascularization in young patients. Ophthalmology 1996;103:1241–1244.
2. Lim JI. Iatrogenic Choroidal Neovascularization. Surv Ophthalmol 1999; 44 (2): 95-111.
3. Pieramici DJ, Gonzalez C, Raja SC. Choroidal Neovascularization Associated With Aplasia of the Optic Nerve. Am J Ophthalmol 2001;132:439-440.
4. Ahmadieh H, Vafi N. Dramatic response of choroidal neovascularization associated with choroidal osteoma to the intravitreal injection of bevacizumab (Avastin). Graefe's Arch Clin Exp Ophthalmol 2007; 245:1731-1733.
5. Stanescu D, Wattenberg S, Cohen SY. Photodynamic Therapy for Choroidal Neovascularization Secondary to Choroidal Nevus. Am J Ophthalmol 2003;136:575-576.
6. Callanan DG, Lewis ML, Byrne SF, Gass JDM. Choroidal neovascularization associated with choroidal nevi. Arch Ophthalmol 1993;111:789-794.
7. Spaide RF, Borodoker N, Shah V. Atypical Choroidal Neovascularization in Radiation Retinopathy. Am J Ophthalmol 2002;133:709-711.
8. Ho AC, Yannuzzi LA, Pisicano K, DeRosa J. The natural history of idiopathic subfoveal choroidal neovascularization. Ophthalmology 1995;102:782–789.
9. Lindblom B, Andersson T. The Prognosis of Idiopathic Choroidal Neovascularization in Persons Younger Than 50 Years of Age. Ophthalmology 1998;105:1816–1820.
10. Iida T, Hagimura N, Kishi S, Shimizu K. Indocyanine Green Angiographic Features of Idiopathic Submacular Choroidal Neovascularization. Am J Ophthalmol 1998;126:70-76.
11. Gass JDM. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol 1994; 118:285-298.
12. Iida T,Hagimura N, Kishi S, Shimizu K. Indocyanine Green Angiographic Features of Idiopathic Submacular Choroidal Neovascularization. Am J Ophthalmol 1998;126:70-76.
13. Iida T, Hagimura N, Sato T, Kishi S. Optical Coherence Tomographic Features of Idiopathic Submacular Choroidal Neovascularization. Am J Ophthalmol 2000;130:763-768.
14. Flaxel CJ, Owens SL, Mulholland B, et al. The use of corticosteroids for choroidal neovascularization in young patients. Eye 1998;12:266 -272.
15. Spaide RF, Martin ML, Slakter J, et al. Treatment of idiopathic choroidal neovascular lesions using photodynamic therapy with verteporfin. Am J Ophthalmol 2002;134:62-68.
16. Chan WM, Lam DS, Wong TH, et al. Photodynamic therapy with verteporfin for subfoveal idiopathic choroidal neovascularization: one-year results from a prospective case series. Ophthalmology 2003;110:2395–2402.
17. Ruiz-Moreno JM, Montero JA, Arias L, Sanabria MR, Coco R, Silva R, Araiz J, Gomez-Ulla F, Garcia-Layana A. Photodynamic therapy in subfoveal and juxtafoveal idiopathic and postinflammatory choroidal neovascularization. Acta Ophthalmol Scand 2006;84(6):743-748.
18. Chen YS, Lin JY, Tseng SY, Tang HF, Lee HJ, Lin YR. Photodynamic therapy of idiopathic subfoveal choroidal neovascularization in Taiwanese patients: a 2-year follow-up. Eye 2008;18 Epub.
19. Giovannini A, Neri P, Mercanti L, Bruè C. Photodynamic treatment versus photodynamic treatment associated with systemic steroids for idiopathic choroidal neovascularisation. Br J Ophthalmol 2007;91(5):620-3. Epub 2007 Jan 3.
20. Chan WM, Lai TY, Lau TT, Lee VY, Liu DT, Lam DS. Combined photodynamic therapy and intravitreal triamcinolone for choroidal neovascularization secondary to punctate inner choroidopathy or of idiopathic origin: one-year results of a prospective series. Retina 2008;28(1):71-80.
Karacorlu M ,Karacorlu S, Ozdemir H. Photodynamic Therapy in Patients with Idiopathic Choroidal Neovascularization. Jpn J Ophthalmol 2004; 48: 422-424.
22. Essex RW, Tufail A, Bunce C, Aylward GW. Two-year results of surgical removal of choroidal neovascular membranes related to non-age-related macular degeneration. Br J Ophthalmol 2007;91(5):649-54.
23. Chan WM, Lai TYY, Liu DTL, Lam DSSC. Intravitreal Bevacizumab (Avastin) for Choroidal Neovascularization Secondary to Central Serous Chorioretinopathy, Secondary to Punctate Inner Choroidopathy, or of Idiopathic Origin. Am J Ophthalmol 2007;143:977-983.
24. Mandal S, Garg S, Venkatesh P, Mithal C, Vohra R, Mehrotra A. Intravitreal bevacizumab for subfoveal idiopathic choroidal neovascularization. Arch Ophthalmol 2007; 125(11):1487-92.
25. Gomi F, Nishida K, Oshima Y, Sakaguchi H, Sawa M, Tsujikawa M, Tano Y. Intravitreal Bevacizumab for Idiopathic Choroidal Neovascularization After Previous Injection With Posterior Subtenon Triamcinolone. Am J Ophthalmol 2007;143:507-509.
26. Kumar A, Prakash G, Singh RP. Transpupillary thermotherapy for idiopathic subfoveal choroidal neovascularization. Acta Ophthalmol Scand 2004;82(2):205-8.
Bibliography CNV post-inflammatory
1. Inagaki M, Harada T, Kiribuchi T, Ohashi T, Majima J. Subfoveal choroidal neovascularization in uveitis. Ophthalmologica 1996;210:229–233.
2. Fine SL, Owens SL, Haller JA, Knox DL, Patz A. Choroidal neovascularization as a late complication of ocular toxoplasmosis. Am J Ophthalmol 1981;91:318-322.
3. Kayazawa F. Subretinal neovascularization associated with presumed toxoplasmic retinochoroidal scar. Ann Ophthalmol 1982;14:812-819.
4. Thorne JE, Wittenberg S, Jab DA, et al. Multifocal Choroiditis with Panuveitis: Incidence of Ocular Complications and of Loss of Visual Acuity. Ophthalmology 2006;113:2310–2316.
5. Moorthy RS, Chong LP, Smith RE, Rao NS. Subretinal neovascular membranes in Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol 1993;116:164-170.
6. Yoser SL, Forster DJ, Rao NA. Systemic viral infections and their retinal and choroidal manifestations. Surv Ophthalmol 1993;37:313-352.
7. Wind BE, Sobol WM. Surgical management of a long-standing subfoveal neovascular membrane secondary to ocular histoplasmosis. Ophthalmic Surg. 1993;24(1):36-39.
8. Adán A, Mateo C, Wolley-Dod C. Surgery for Subfoveal Choroidal Neovascularization in Toxoplasmic Retinochoroiditis. Am J Ophthalmol 2003;135:386-387.
9. Wang KL, Kansal S, Pulido JS. Photodynamic Therapy for the Treatment of Choroidal Neovascularization Secondary to Rubella Retinopathy. Am J Ophthalmol 2002;134:790-792.
10. Farah ME, Costa RA, Muccioli C, Guia TA, Belfort R Jr. Photodynamic Therapy With Verteporfin for Subfoveal Choroidal Neovascularization in Vogt-Koyanagi-Harada Syndrome. Am J Ophthalmol 2002;134:137-139.
11. Spaide RF, Freund KB, Slakter J, Sorenson J, Yannuzzi LA, Fisher Y. Treatment of subfoveal choroidal neovascularization associated with multifocal choroiditis and panuveitis with photodynamic therapy. Retina 2002;22:545-549.
12. Ruiz-Moreno JM, Montero JA, Arias L, Sanabria MR, Coco R, Silva R, Araiz J, Gomez-Ulla F, Garcia-Layana A. Photodynamic therapy in subfoveal and juxtafoveal idiopathic and postinflammatory choroidal neovascularization. Acta Ophthalmol Scand 2006;84(6):743-748.
13. Fong KC, Thomas D, Amin K, Inzerillo D, Horgan SE. Photodynamic therapy combined with systemic corticosteroids for choroidal neovascularisation secondary to punctate inner choroidopathy. Eye 2008;22(4):528-533. Epub 2007 Jan 19.
14. Postelmans L, Pasteels B, Coquelet P, et al. Photodynamic therapy for subfoveal classic choroidal neovascularization related to punctate inner choroidopathy (PIC) or presumed ocular histoplasmosis-like syndrome (POHS-like). Ocul Immunol Inflamm 2005;13:361-366.
15. Lim JI, Flaxel CJ, LaBree L. Photodynamic therapy for choroidal neovascularisation secondary to inflammatory chorioretinal disease. Ann Acad Med Singapore 2006;35(3):198-202.
16. Rogers AH, Duker JS, Nichols N, Baker BJ. Photodynamic therapy of idiopathic and inflammatory choroidal neovascularization in young adults. Ophthalmology 2003;110:1315–1320.
17. Chan WM, Lai TY, Lau TT, Lee VY, Liu DT, Lam DS. Combined photodynamic therapy and intravitreal triamcinolone for choroidal neovascularization secondary to punctate inner choroidopathy or of idiopathic origin: one-year results of a prospective series. Retina 2008;28(1):71-80.
18. Izumi-Nagai K, Nagai N, Ozawa Y, Mihara M, Ohsugi Y, Kurihara T, Koto T, Satofuka S, Inoue M, Tsubota K, Okano H, Oike Y, Ishida S. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol 2007;170(6):2149-2158.
19. Adán A, Mateo C, Navarro R, Bitrian E, Casaroli-Marano RP. Intravitreal bevacizumab (avastin) injection as primary treatment of inflammatory choroidal neovascularization. Retina 2007;27(9):1180-1186.
Dr. Carmelo Chines
Direttore responsabile
