We interviewed Dr. Florian Sennlaub, Director of Team 14: 'Inflammation, dégénérescence et remodellage vasculaire' at the Institut de la Vision in Paris, on the latest news on inflammation and AMD.
Are there therefore new targets towards which pharmacological research should be directed?
Recent studies suggest that mediators of inflammation play an important role in the pathophysiology of AMD. Drusen (whitish subretinal deposits) have been shown to contain immune system molecules such as immune complexes, complement factors, Major Histocompatibility Complex (MAHC) and amyloid oligomers. On the other hand, AMD is associated with a polymorphism that attenuates factor H function and with the loss of factor H inhibitory activity, the complement system becomes hyperactive during AMD. However, it remains unclear how complement hyperactivation contributes to the pathogenesis of AMD.
Microglial cells, resident macrophages in the central nervous system, are localised in the inner retina [1] and are normally absent from the photoreceptor layer and subretinal space located between the retinal pigment epithelium (EPR) and the retina. In AMD, macrophages become activated and accumulate [2, 3] in the subretinal space [1, 4]. This accumulation has been interpreted as a secondary effect. Macrophages would be recruited to clean the photoreceptor layer of dead cells.
[caption id="attachment_759" align="aligncenter" width="267" class=" "]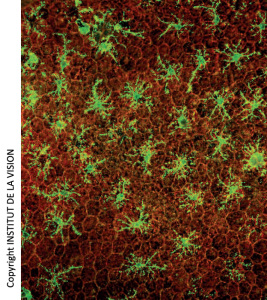 Subretinal macrophages on horizontal mounts of Retinal Pigmented Epithelium (EPR). In red, the cells of the EPR (honeycomb). In green, the macrophages.[/caption]
Subretinal macrophages on horizontal mounts of Retinal Pigmented Epithelium (EPR). In red, the cells of the EPR (honeycomb). In green, the macrophages.[/caption]
Over the past decade, numerous studies have addressed the role of chemokines, small secreted proteins that recruit macrophages in inflammatory diseases. The chemokines CCL2 and CX3CL1 play a key role in the recruitment and activation of monocytes, macrophages and microglial cells [5, 6].
The chemokine CCL2 is released through tissue injury and participates in the recruitment of circulating inflammatory monocytes that overexpress the CCR2 receptor. The recruited inflammatory monocytes rapidly reduce CCR2 expression and differentiate into inflammatory macrophages (M?). The inflammatory macrophage (M?) secretes a number of bactericidal factors that can also have a neurotoxic effect. Later, the M? acquire a less aggressive phenotype that promotes angiogenesis and healing, before disappearing or remaining in the tissue for a long time as a tissue macrophage.
CX3CL1 is an atypical chemokine: it is a transmembrane protein with an integrin-like function. In the eye, it is constitutively expressed by retinal and EPR neurons. Its receptor, CX3CR1, is expressed in large quantities by microglial cells [1, 7].
CX3CL1/CX3CR1 signalling induces a supportive phenotype in Microglial Cells (MCs). CX3CL1, cleaved from the membrane by proteases into a soluble form, can develop chemotactic properties [8].
A macrophage that is fully differentiated but derived from an infiltrating monocyte can become very similar to a microglial cell, present in the retina after foetal development, both in terms of its morphology and the proteins it expresses.
Do these studies have any bearing on the pathogenesis of AMD?
A few years ago we observed to our great surprise that mice lacking the Cx3cr1 gene (Cx3cr1-/ mice) present a spontaneous age-dependent accumulation of inflammatory cells (M? or MCs) in the subretinal space. This accumulation occurs in the absence of initial photoreceptor or EPR degeneration [1]. The results contrast sharply with the behaviour of M? in peripheral inflammatory tissue, which shows a deficit of accumulation in lesion areas following the absence of Cx3cr1 [6].
[caption id="attachment_760" align="aligncenter" width="143" class=" "]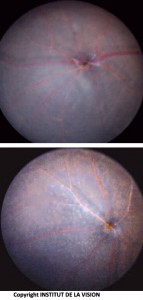 Bottom of the eye of knockout and control mice. Subretinal macrophages (knockout mouse) are visible as small white dots.[/caption]
Bottom of the eye of knockout and control mice. Subretinal macrophages (knockout mouse) are visible as small white dots.[/caption]
In Cx3cr1-/- mice, the prolonged presence of M?/MCs in the subretinal space is associated with excessive phagocytosis of the outer segments of the photoreceptors by M?/MCs, resulting in the accumulation of intracellular lipids [9, 1].
What, in brief, are the objectives and potential therapeutic developments of the activities conducted by your research group?
The research carried out in our laboratory attempts to identify the mechanisms of macrophage recruitment in the subretinal space, the factors that favour their survival and thus their accumulation, and the neurotoxic and angiogenic factors secreted by the inflammatory cells.
This innovative concept will enable us to develop new drug therapies that aim to prevent the accumulation of macrophages and/or their pathogenic factors. In animals, these treatments are able to prevent both photoreceptor degeneration and neovascularisation. In humans, they could constitute a new therapeutic treatment for atrophic and wet forms of AMD in the relatively near future.
Bibliography
1) Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F (2007) CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest 117:2920-2928.
2) Penfold PL, Liew SC, Madigan MC, Provis JM (1997) Modulation of major histocompatibility complex class II expression in retinas with age-related macular degeneration. Invest Ophthalmol Vis Sci 38:2125-2133.
3) van der Schaft TL, Mooy CM, de Bruijn WC, de Jong PT (1993) Early stages of age-related macular degeneration: an immunofluorescence and electron microscopy study. Br J Ophthalmol 77:657-661.
4) Gupta N, Brown KE, Milam AH (2003) Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res 76:463-471.
5) Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z (2008) Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117:1649-1657.
6) Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debre P, Tedgui A, Murphy PM, Mallat Z (2003) Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation 107:1009-1016.
7) Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S (2006) Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci 47:3595-3602.
8) Ransohoff RM (2009) Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31:711-721.
9) Raoul W, Feumi C, Keller N, Lavalette S, Houssier M, Behar-Cohen F, Combadiere C, Sennlaub F (2008) Lipid-bloated subretinal microglial cells are at the origin of drusen appearance in CX3CR1-deficient mice. Ophthalmic Res 40:115-119.
10) Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF (2010) Prevalence and Significance of Subretinal Drusenoid Deposits (Reticular Pseudodrusen) in Age-Related Macular Degeneration. Ophthalmology.
11) Rudolf M, Malek G, Messinger JD, Clark ME, Wang L, Curcio CA (2008) Sub-retinal drusenoid deposits in human retina: organisation and composition. Exp Eye Res 87:402-408.
12) Cohen SY, Dubois L, Tadayoni R, Delahaye-Mazza C, Debibie C, Quentel G (2007) Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol 91:354-359.
13) Raoul W, Keller N, Rodero M, Behar-Cohen F, Sennlaub F, Combadiere C (2008) Role of the chemokine receptor CX3CR1 in the mobilisation of phagocytic retinal microglial cells. J Neuroimmunol 198:56-61.
14) Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT (2009) Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS One 4:e7945.
15) Roque RS, Rosales AA, Jingjing L, Agarwal N, Al-Ubaidi MR (1999) Retina-derived microglial cells induce photoreceptor cell death in vitro. Brain Res 836:110-119.
16) Nakazawa T, Hisatomi T, Nakazawa C, Noda K, Maruyama K, She H, Matsubara A, Miyahara S, Nakao S, Yin Y, Benowitz L, Hafezi-Moghadam A, Miller JW (2007) Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc Natl Acad Sci U S A 104:2425-2430.
For more information contact:
Florian Sennlaub
Institut de la Vision
17, rue Moreau
75012 Paris - France
E.mail: Florian.sennlaub[at]inserm.fr
Web site: www.institut-vision.org
Dr. Carmelo Chines
Direttore responsabile


