Latest updates at the 2017 AAO Congress
New hope for patients suffering from hereditary maculopathies, according to results presented at 121mo Congress of the American Academy of Ophthalmology (AAO), which has just concluded in New Orleans (USA - 11-14 November 2017).
The patients enrolled in the study were suffering from Leber Congenital Amaurosis (LCA), a rare genetic disorder that, while other hereditary maculopathies tend to manifest around the age of 20-30 years, arises in very early childhood and progresses slowly, eventually leading to complete blindness.
For this, as for other hereditary maculopathies, there is currently no medical therapy capable of counteracting its course.
As early as 1997, the cause of LCA was identified as certain mutations in the RPE65 genewhich prevent the synthesis of a protein that enables the proper uptake of vitamin A by retinal pigment epithelium cells. The aim was therefore to identify the DNA segment corresponding to the normal RPE65 gene and to insert it into a vector consisting of a virus (AAV: adeno-associated viral vector), which is injected under the retina where it releases the healthy genetic segment. In this way, the RPE65 protein can be correctly synthesised and carry out its activity on the visual receptors.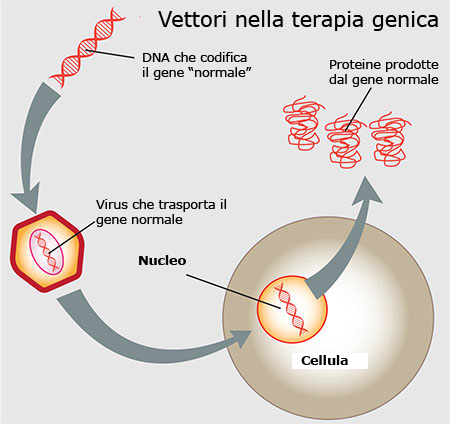
Dr. Stephen R. Russell of the IOWA University research team, which is testing this pioneering gene therapy, reports that in the phase III clinical trial 27 of the 29 patients treated (the 93%) achieved a significant improvement in their visual acuity, such that they were able to move around a maze with low to medium illumination on their own. There was also an improvement in light sensitivity and peripheral vision, which are the two types of visual impairment affecting these patients. Clearly, patients do not regain normal visual function, but they can distinguish shapes and perceive light. It is not possible to state with certainty that the results achieved will persist over time, but in the treated patients they were maintained for about two years.
The proposed gene therapy is currently being examined by the Food and And Drug Administration (FDA) and in October theadvisory committee was unanimously in favour of the treatment, which could therefore receive approval in early 2018.
It would be the first gene therapy authorised for the treatment of a hereditary maculopathy and could pave the way for similar therapies for other maculopathies due to genetic mutations, such as retinitis pigmentosa or Stargardt's maculopathy.
For more information see The Research Road: Gene Therapy for Leber Congenital Amaurosis of the National Eye Institute (NEI)
Dr. Carmelo Chines
Direttore responsabile
